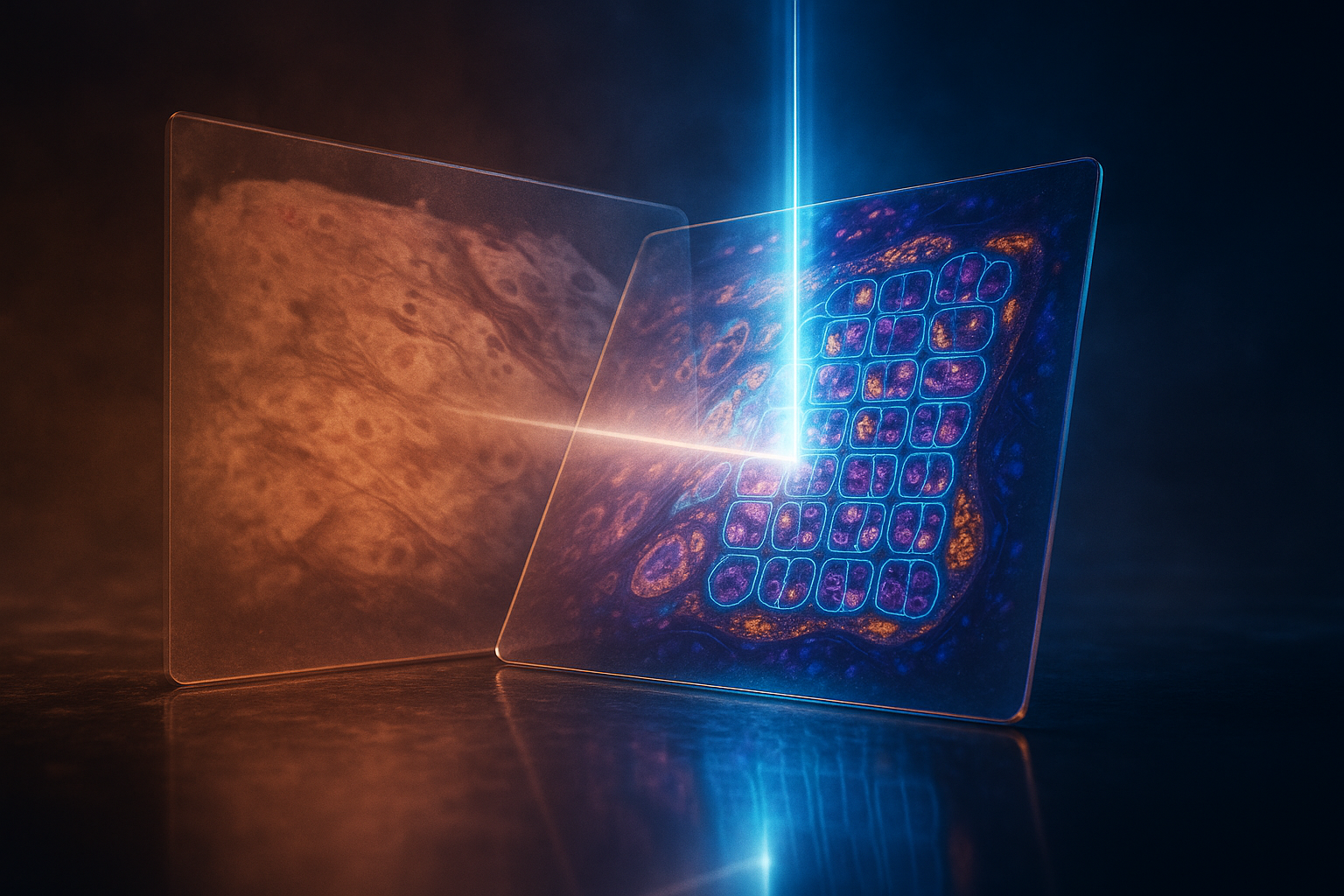
Opening — Why this matters now
Modern pathology is going digital at breakneck speed, yet the transition hides a deceptively analog bottleneck: aligning images that never quite match. Tissue slides stained with hematoxylin-eosin, immunofluorescence, or PAS may originate from the same biopsy—but their digital twins rarely align pixel-to-pixel. This mismatch thwarts the holy grail of computational pathology: integrating structure, function, and molecular signals into one coherent visual map.
The newly proposed CORE (Cell-level Coarse-to-fine Registration Engine) offers a pragmatic breakthrough. It unites multi-stained whole-slide images (WSIs) through a two-stage framework that’s not only faster but more biologically faithful than anything before. In short, it makes “multimodal” mean “mutually comprehensible.”
Background — Context and prior art
Image registration—the process of aligning two or more images into a shared coordinate space—has always been central to medical imaging. But in histopathology, where each image can exceed a gigapixel, conventional algorithms falter. Deformations caused by tissue slicing, staining, and scanning introduce nonlinear distortions. Worse, different stains highlight distinct biological features: what looks like structure in H&E may vanish in mIF.
Earlier methods such as HistokatFusion, VALIS, and DeeperHistReg have pushed registration forward, but each remains bound by either computational cost or modality bias. Deep-learning-based extractors like SuperPoint and SuperGlue improved local feature matching, yet struggled to maintain global consistency. In practice, even state-of-the-art systems required hours per slide and manual tuning for stain combinations.
Analysis — What CORE does differently
CORE reimagines the pipeline as a coarse-to-fine negotiation between two scales of biological truth: tissue morphology and cellular architecture.
| Stage | Scale | Key Technique | Function |
|---|---|---|---|
| Coarse | Low (0.625×–1.25×) | Prompt-based tissue mask + deep feature alignment (XFeat) | Rapid global registration using morphology |
| Fine | High (20×) | Shape-aware nuclei-level point-set registration + CPD deformation | Local, cell-accurate correction |
1. Coarse Registration
CORE begins by removing distractions. Using Florence-2 and SAM, it segments tissue from artefacts via “prompted” segmentation prompts (like tissue, stain, histology). Then, the TriMorph step aligns slides through center-of-mass, scale, and rotation estimation—an elegant triad of geometric logic. XFeat then extracts up to 16,000 semi-dense keypoints per slide, feeding a dense feature-matching routine that’s both fast and stain-agnostic.
The result? Coarse registration completes in roughly 15 seconds, outperforming peers by an order of magnitude.
2. Fine, Shape-Aware Registration
After global alignment, CORE dives into the microscopic world. Each nucleus becomes a landmark. The system detects nuclei via adaptive thresholding and morphological operations—trading deep learning’s fragility for classic computer vision’s reliability. Each nucleus is represented as a point with both spatial and shape descriptors, enabling a hybrid metric that balances position ($\gamma = 0.3$) and morphology.
Then comes Coherent Point Drift (CPD), a non-rigid alignment that warps one cell constellation into another—without anatomical absurdities. Deformation fields are regularized to prevent local folding, ensuring biological plausibility.
The process culminates in near-perfect cellular correspondence across stains—a computational feat once thought unattainable.
Findings — How CORE performs
CORE was benchmarked on five datasets spanning public and private cohorts (ACROBAT, ANHIR, HYRECO, Multi-IHC CRC, and REACTIVAS). Across all, it consistently achieved lower registration error and shorter runtimes.
| Dataset | Best rTRE ↓ | Avg. Time per Slide | Key Competitor |
|---|---|---|---|
| ACROBAT | 139 µm | 14 s | DeeperHistReg (140 µm, 70 s) |
| ANHIR | 0.0021 | 28 min (fine) | HistokatFusion (0.0027, 9.6 s coarse only) |
| HYRECO | 4.35 µm | 47 min (fine) | DFBR (30 µm) |
| Multi-IHC CRC | 0.0011 | 36 min | DFBR (0.0031) |
| REACTIVAS (mIF) | 0.846 µm | 25 min | DeeperHistReg (3.67 µm) |
Performance isn’t the only story—robustness is. CORE handled cross-stain pairs like H&E–mIF and PAS–mIF, where earlier methods often failed outright. The coarse stage alone was often “good enough” for downstream workflows, while the fine stage unlocked true nuclei-level mapping.
Implications — Why this changes digital pathology
CORE’s hybrid logic—deep features for speed, nuclei landmarks for accuracy—marks a turning point. It bridges computational pathology’s two camps: the AI maximalists chasing pure deep learning, and the traditionalists favoring handcrafted morphology.
For hospitals, this means scalable multimodal slide integration across staining protocols without manual adjustment. For biotech, it enables cross-marker correlation at subcellular precision—fueling insights in oncology, renal pathology, and immunotherapy. And for AI developers, CORE’s open-source release offers a reproducible, domain-agnostic benchmark.
The cost? Time. The fine registration stage is slower, but precision at the cellular level rarely comes cheap. Future versions could hybridize classical and neural segmentation or introduce self-supervised stain adaptation to further cut runtime.
Conclusion — From slide to system
In pathology, “alignment” has always been both a technical and philosophical pursuit—how to make heterogeneous evidence speak a common visual language. CORE achieves this by aligning not just images but epistemologies: combining statistical elegance, deep learning efficiency, and biological realism.
At a time when medical AI risks becoming a black box, CORE is refreshingly interpretable. Its mathematics are transparent, its logic modular, its results reproducible. It doesn’t just automate the microscope—it teaches it to agree with itself.
Cognaptus: Automate the Present, Incubate the Future.